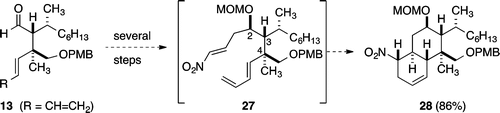
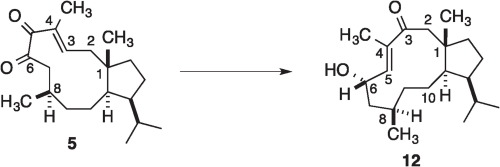
Strategies and Discoveries Leading to the Synthesis of Trichoaurantianolide Natural Products, in Strategies and Tactics in Organic Synthesis
D. R. Williams, Strategies and Discoveries Leading to the Synthesis of Trichoaurantianolide Natural Products, in Strategies and Tactics in Organic Synthesis, Vol. 15, Harmata, M., Ed.; Elsevier Limited, 2021, in press.
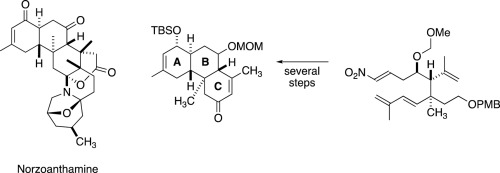
Studies Toward Norzoanthamine: Ireland–Claisen Rearrangements of α,β-Unsaturated Esters in a Stereocontrolled Synthesis of Trans-Fused 2-Cyclohexen-1-ones
D. R. Williams, P. T. Gladen, and S. Patnaik, Studies Toward Norzoanthamine: Ireland–Claisen Rearrangements of ,-Unsaturated Esters in a Stereocontrolled Synthesis of Trans-Fused 2-Cyclohexen-1-ones, Tetrahedron 2021, 95, 132354 (1–15), (Dale Boger Special Issue).
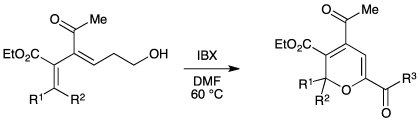
IBX Oxidations for the Synthesis of Substituted 2H-Pyrans
D. R. Williams, N. Haddadpour, S. A. Bawel, and S. Maier, IBX Oxidations for the Synthesis of Substituted 2H-Pyrans, Heterocycles 2021, 103, 707–713, (Yasuyuki Kita Special Issue).
Compounds from Plantar Foot Sweat, Nesting Material, and Urine Show Strain Patterns Associated with Agonistic and Affiliative Behaviors in Group Housed Male Mice
A. J. Barabas, H. A. Soini, M. V. Novotny, D. R. Williams, J. A. Desmond J. R. Lucas, M. A. Erasmus, H.-W. Cheng, and B. N. Gaskill, Compounds from Plantar Foot Sweat, Nesting Material, and Urine Show Strain Patterns Associated with Agonistic and Affiliative Behaviors in Group Housed Male Mice, Mus musculus, PLoS ONE, 2021, 16, e0251416 (1–29).
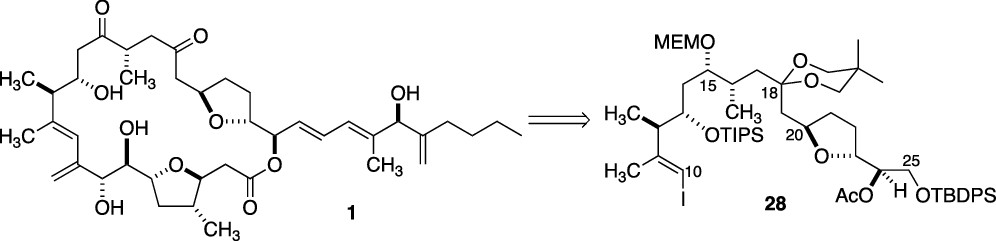
Studies of the Enantiocontrolled Synthesis of the C(10)–C(25) Subunit of Amphidinolide C
D. R. Williams, R. De, M. W. Fultz, D. A. Fischer, Á. Morales-Ramos, and D. Rodríguez-Reyes, Studies of the Enantiocontrolled Synthesis of the C(10)–C(25) Subunit of Amphidinolide C, Org. Lett. 2020, 22, 4118–4122
Synthesis and Biological Activity of Two Volatile Cyclic Dipeptides in a Terrestrial Vertebrate
C. Romero-Diaz, S. M. Campos, M. A. Herrmann, K. N. Lewis, D. R. Williams, H. A. Soini, M. V. Novotny, D. K. Hews, E. P. Martins, Structural Identification, Synthesis and Biological Activity of Two Volatile Cyclic Dipeptides in a Terrestrial Vertebrate, Scientific Reports 2020, 10, 4303 (1–10).
6′-Methoxy Raloxifene-Analog Enhances Mouse Bone Properties with Reduced Estrogen Binding
K. M. Powell, A. P. Brown, C. G. Scaggs, A. Pulliam, A. Berman, P. Deosthale, L. I. Plotkin M. R. Allen, D. R. Williams, J. M. Wallace, 6′-Methoxy Raloxifene-Analog Enhances Mouse Bone Properties with Reduced Estrogen Binding, Bone Reports 2020, 12, 100246 (1-9).
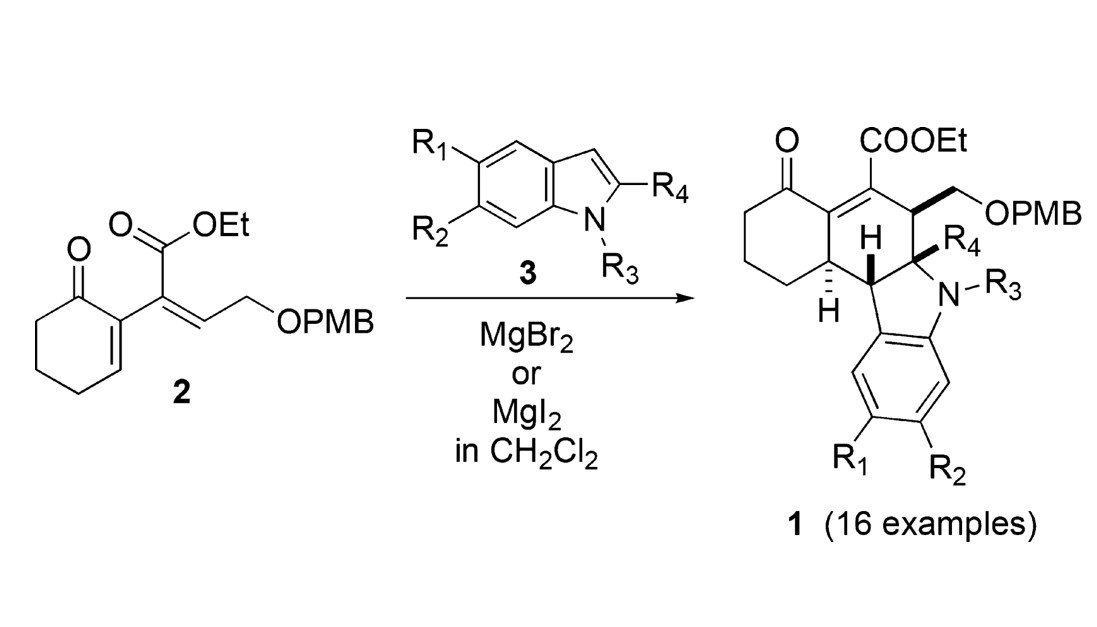
A Stereoselective Michael–Mannich Annelation Strategy for the Efficient Construction of Novel Tetrahydrocarbazoles
D. R. Williams, S. A. Bawel and R. N. Schaugaard, A Stereoselective Michael–Mannich Annelation Strategy for the Efficient Construction of Novel Tetrahydrocarbazoles, Org. Lett. 2017, 19, 5098–5101.

The Development of Strategies and Tactics Toward the Pseudopterosin Natural Products: Ileabethoxazole As a Platform for Target-Inspired Discovery
D. R. Williams, The Development of Strategies and Tactics Toward the Pseudopterosin Natural Products: Ileabethoxazole As a Platform for Target-Inspired Discovery, in Strategies and Tactics in Organic Synthesis, Vol. 13, Harmata, M., Ed.; Elsevier Limited, 2017, pp. 135–160.
General Methodology for the Preparation of Unsymmetrical α-Linked Bisenones via Ligandless Cross-Coupling Reactions
D. R. Williams and S. A. Bawel, General Methodology for the Preparation of Unsymmetrical a-Linked Bisenones via Ligandless Cross-Coupling Reactions, Org. Lett. 2017, 19, 1730–1733.
Studies of Azetidin-2-one as a Reactive Enolate Synthon of β-Alanine for Condensations with Aldehydes and Ketones
D. R. Williams, A. F. Donnell, D. C. Kammler, S. A. Ward and Levin Taylor, IV, Studies of Azetidin-2-one as a Reactive Enolate Synthon of β-Alanine for Condensations with Aldehydes and Keontes, J. Org, Chem. 2016, 81, 10463–10475.
The Preparation and Reactivity of 2-Bromo-3-(tri-n-butylstannyl)-1-Propene
D. R. Williams, A. A. Shaw, D. A. Brooks and N. Zorn, The Preparation and Reactivity of 2-Bromo-3-(tri-n-butylstannyl)-1-Propene, Synlett 2016, 27, 2836-2840.
Studies Toward Australifungin. A Synthesis Dilemma of Regioselective Keto-Enol Tautomerization
D. R. Williams, J. C. Klein, L. C. Kopel, N. Nguyen, and D. J. Tantillo, Studies Toward Australifungin. A Synthesis Dilemma of Regioselective Keto-Enol Tautomerization, Org. Lett. 2016, 18, 424-427.
Intramolecular Diels–Alder (IMDA) Studies toward the Synthesis of Australifungin. Stereocontrol in the Acetate Aldol Reaction of β,β’-Branched Aldehydes
D. R. Williams and J. C. Klein, Intramolecular Diels–Alder (IMDA) Studies toward the Synthesis of Australifungin. Stereocontrol in the Acetate Aldol Reaction of β,β‘-Branched Aldehydes, Org. Lett. 2016, 18, 420-423.
Total Synthesis of Amphidinolide K, a Macrolide that Stabilizes F-Actin
D. Sánchez, T. Andreou, A. M. Costa, K. G Meyer, D. R. Williams, I. Barasoain, J. Fernando Díaz, D. Lucena, J. Vilarrasa, Total Synthesis of Amphidinolide K, a Macrolide that Stabilizes F-Actin, J. Org. Chem. 2015, 80, 8511–8519. (Highlight Paper)
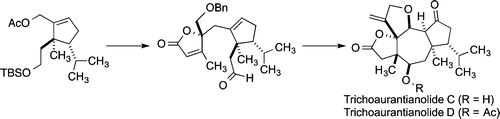
Total Synthesis of Neodolastane Diterpenes Trichoaurantianolides C and D
D. R. Williams, P. T. Gladen and J. R. Pinchman, Total Synthesis of Neodolastane Diterpenes Trichoaurantianolides C and D, J. Org. Chem. 2015, 80, 5474–5493.
Conformational Effects and Stereocontrol in Synthesis Studies of Medium-ring Dolabellane Carbocycles
D. R. Williams, L. A. Robinson and S. A. Bawel, Conformational Effects and Stereocontrol in Synthesis Studies of Medium-ring Dolabellane Carbocycles, Tetrahedron Lett. 2015, 56, 3200–3203. NIHMSID652558
![Total Synthesis of (+)-Ileabethoxazole. Studies of an Intramolecular Iron-Mediated Pauson–Khand [2+2+1] Carbocyclization](https://williams.lab.indiana.edu/wp-content/plugins/ezpublications//assets/images/default-pic.jpg)
Total Synthesis of (+)-Ileabethoxazole. Studies of an Intramolecular Iron-Mediated Pauson–Khand [2+2+1] Carbocyclization
D. R. Williams and A. A. Shah, Total Synthesis of (+)-Ileabethoxazole. Studies of an Intramolecular Iron-Mediated Pauson–Khand [2+2+1] Carbocyclization, J. Am. Chem. Soc. 2014, 136, 8829–8836.

A Stereocontrolled Synthesis of the Tricyclic ABC Ring System of Daphnicyclidin A
D. R. Williams, P. K. Mondal, S. A. Bawel and P. P. Nag, A Stereocontrolled Synthesis of the Tricyclic ABC Ring System of Daphnicyclidin A, Org. Lett. 2014, 16, 1956–1959.

Stereocontrol in Asymmetric SE’ Reactions of γ-Substituted α,β-Unsaturated Aldehydes
D. R. Williams, B. A. Atwater, S. A. Bawel, P. Ke, O. Gutierrez, and D. J. Tantillo, Stereocontrol in Asymmetric SE‘ Reactions of γ-Substituted α,β-Unsaturated Aldehydes, Org. Lett. 2014, 16, 468–471.

Studies for the Total Synthesis of Amphidinolide P
D. R. Williams, B. J. Myers, L. Mi, R. J. Binder, Studies for the Total Synthesis of Amphidinolide P, J. Org. Chem. 2013, 78, 4762–4778. [PDF]

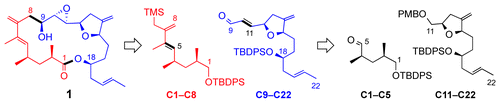
Studies of Neodolastanes. Synthesis of the Tricyclic Core of the Trichoaurantianolides
D. R. Williams and J. R. Pinchman, Studies of Neodolastanes. Synthesis of the Tricyclic Core of the Trichoaurantianolides, Can. J. Chem. 2013, 91, 21–37. (D. Clive Tribute Issue).

Studies of Iron-Mediated Pauson—Khand Reactions of 1,1-Disubstituted-Allenylsilanes: Mechanistic Implications for a Reactive Three-Membered Iron Metallacycle
D. R. Williams, A. A. Shah, S. Mazumder, and M.-H. Baik, Studies of Iron-Mediated Pauson—Khand Reactions of 1,1-Disubstituted-Allenylsilanes: Mechanistic Implications for a Reactive Three-Membered Iron Metallacycle, Chem. Sci. 2013, 4, 238-247.

A Bidirectional SE’ Strategy for 1,5-syn and 1,5-anti Stereocontrol Toward the Synthesis of Complex Polyols
D. R. Williams, C. D. Claeboe, and N. Zorn, A Bidirectional SE‘ Strategy for 1,5-syn and 1,5-anti Stereocontrol Toward the Synthesis of Complex Polyols, Org. Lett. 2012, 14, 3866–3869.[PDF]

Investigation of Scents on Cheeks and Foreheads of Large Felines in Connection to the Facial Marking Behavior
H. A. Soini, S. U. Linville, D. Wiesler, A. L. Posto, D. R. Williams, M. V. Novotny, Investigation of Scents on Cheeks and Foreheads of Large Felines in Connection to the Facial Marking Behavior, J. Chem. Ecol. 2012, 38, 145–156.

Enantioenriched Formation of Synthons of 1,5-Hexadiene Bis-Epoxides for Construction of Nonracemic cis- and trans-2,5-Disubstituted Tetrahydrofurans
D. R. Williams and R. De, Enantioenriched Formation of Synthons of 1,5-Hexadiene Bis-Epoxides for Construction of Nonracemic cis– and trans-2,5-Disubstituted Tetrahydrofurans, Heterocycles, 2012, 84, 385–391 (A. Padwa Special Issue).
![Silane, 1,1'-(Chloromethylene)bis[1,1,1]-trimethyl-](https://williams.lab.indiana.edu/wp-content/plugins/ezpublications//assets/images/default-pic.jpg)
Silane, 1,1′-(Chloromethylene)bis[1,1,1]-trimethyl-
D. R. Williams and M. J. Walsh, Silane, 1,1‘-(Chloromethylene)bis[1,1,1]-trimethyl-, in Handbook of Reagents for Organic Synthesis, Reagents for Silicon-Mediated Organic Synthesis, Fuchs, P. L., Ed.; John Wiley & Sons Ltd.: Chichester, West Sussex, UK, 2011, pp. 461–463.

Studies for the Enantiocontrolled Preparation of Substituted Tetrahydropyrans: Applications for the Synthesis of Leucascandrolide A Macrolactone
D. R. Williams, S. V. Plummer and S. Patnaik, Studies for the Enantiocontrolled Preparation of Substituted Tetrahydropyrans: Applications for the Synthesis of Leucascandrolide A Macrolactone, Tetrahedron, 2011, 67, 5083–5097. [PDF]

Studies of Intramolecular Diels–Alder Reactions of Nitroalkenes for the Stereocontrolled Synthesis of trans-Decalin Ring Systems
D. R. Williams, J. C. Klein, N. S. C. Chow, Studies of Intramolecular Diels–Alder Reactions of Nitroalkenes for the Stereocontrolled Synthesis of trans-Decalin Ring Systems, Tetrahedron Lett. 2011, 52, 2120–2123. [PDF]

Efficient Suzuki and Stille Reactions for Regioselective Strategies of Incorporation of the 1,3-Oxazole Heterocycle: Mild Desulfonylation for the Synthesis of C-4 and C-5 Monosubstituted Oxazoles
D. R. Williams and L. Fu, Efficient Suzuki and Stille Reactions for Regioselective Strategies of Incorporation of the 1,3-Oxazole Heterocycle: Mild Desulfonylation for the Synthesis of C-4 and C-5 Monosubstituted Oxazoles, Synlett, 2010, 1641–1646. [PDF]

Regioselective Formation of 1,1-Disubstituted Allenylsilanes via Cross-Coupling Reactions of 3-Tri-n-butylstannyl-1-trimethylsilyl-1-propyne
D. R. Williams and A. A. Shah, Regioselective Formation of 1,1-Disubstituted Allenylsilanes via Cross-Coupling Reactions of 3-Tri-n-butylstannyl-1-trimethylsilyl-1-propyne, Chem. Commun. 2010, 46, 4297–4299. [PDF]

Methodology for the Synthesis of Substituted 1,3-Oxazoles
D. R. Williams and L. Fu, Methodology for the Synthesis of Substituted 1,3-Oxazoles, Synlett, 2010, 591–594.[PDF]

General Methodology for the Preparation of 2,5-Disubstituted-1,3-Oxazoles
D. R. Williams and L. Fu, General Methodology for the Preparation of 2,5-Disubstituted-1,3-Oxazoles, Org. Lett. 2010, 12, 808–811.[PDF]

A General Preparation of (Z)-1-Fluorostilbene Derivatives for the Design of Conformationally Restricted Peptidomimetics
D. R. Williams, M. W. Fultz, T. E. Christos, and J. S. Carter, A General Preparation of (Z)-1-Fluorostilbene Derivatives for the Design of Conformationally Restricted Peptidomimetics, Tetrahedron Lett. 2010, 51, 121–124. [PDF]

Studies of Xenicane Diterpenes. A Stereocontrolled Total Synthesis of 4-Hydroxydictyolactone
D. R. Williams, M. J. Walsh, and N. A. Miller, Studies of Xenicane Diterpenes. A Stereocontrolled Total Synthesis of 4-Hydroxydictyolactone, J. Am. Chem. Soc. 2009, 131, 9038–9045.[PDF]

The Ugi Multicomponent Reaction
D. R. Williams and M. J. Walsh, The Ugi Multicomponent Reaction, in Name Reactions for Homologations – Part II, Li, J. J. and Corey, E. J., Eds.; Wiley & Sons: Hoboken, NJ, 2009, pp. 786–805.

The Passerini Reaction
D. R. Williams and J. C. Klein, The Passerini Reaction, in Name Reactions for Homologations – Part II, Li, J. J. and Corey, E. J., Eds.; Wiley & Sons: Hoboken, NJ, 2009, pp. 765–785.

Claisen and Related Rearrangements
D. R. Williams and P. P. Nag, Claisen and Related Rearrangements, in Name Reactions for Homologations – Part II, Li, J. J. and Corey, E. J., Eds.; Wiley & Sons: Hoboken, NJ, 2009, pp. 33–87.

Studies for the Synthesis of Marine Natural Products
D. R. Williams, M. J. Walsh, C. D. Claeboe, and N. Zorn, Studies for the Synthesis of Marine Natural Products, Pure Appl. Chem. 2009, 81, 181–194. [PDF]

N,N-Dimethylacetamide Dimethyl Acetal (Update)
D. R. Williams, M. W. Fultz, N,N-Dimethylacetamide Dimethyl Acetal (Update), in Electronic Encyclopedia of Reagents for Organic Synthesis, Fuchs, P., Ed.; John Wiley & Sons Ltd.: Sussex, 2009.

The Development of Strategies for the Synthesis of Peloruside A – a Novel Antitumor Agent
D. R. Williams, P. P. Nag and N. Zorn, The Development of Strategies for the Synthesis of Peloruside A – a Novel Antitumor Agent, Curr. Opin. Drug Discovery Dev. 2008, 11, 251–271.[PDF]

Reactions of SE’ Substitution for Organostannanes in Organic Synthesis
D. R. Williams, P. P. Nag, Reactions of SE‘ Substitution for Organostannanes in Organic Synthesis, in Tin Chemistry – Fundamentals, Frontiers and Applications, Davies, A., Gielen, M., Pannell, K. and Tiekink, E., Eds.; John Wiley & Sons, Ltd.: Chichester, 2008, pp. 515–560.

Dioxobis(2,4-pentanedionato)molybdenum, MoO2(acac)2
D. R. Williams, J. C. Klein, Dioxobis(2,4-pentanedionato)molybdenum, MoO2(acac)2, in Electronic Encyclopedia of Reagents for Organic Synthesis, Molander, G., Ed.; John Wiley & Sons Ltd.: Sussex, 2008.

Synthesis Studies of Dolabellanes and Transannular Processes Leading to Related Diterpenes
D. R. Williams, Synthesis Studies of Dolabellanes and Transannular Processes Leading to Related Diterpenes, in Strategies and Tactics in Organic Synthesis, Vol. 7, Harmata, M., Ed.; Elsevier: Amsterdam, 2008, pp. 243–267.
![A General Scheme for the Preparation of Functionalized 8-Oxa-6-azabicyclo[3.2.1]octanes](https://williams.lab.indiana.edu/wp-content/plugins/ezpublications//assets/images/default-pic.jpg)
A General Scheme for the Preparation of Functionalized 8-Oxa-6-azabicyclo[3.2.1]octanes
D. R. Williams, S. Patnaik and G. S. Cortez, Studies of Zoanthamine Alkaloids. A General Scheme for the Preparation of Functionalized 8-Oxa-6-azabicyclo[3.2.1]octanes, Heterocycles, 2007, 72, 213–219 (Y. Kishi Special Issue).

Strategies for the Synthesis of Fusicoccanes via Nazarov Reactions of Dolabelladienones. Total Synthesis of (+)-Fusicoauritone
D. R. Williams, L. A. Robinson, C. R. Nevill and J. P. Reddy, Strategies for the Synthesis of Fusicoccanes via Nazarov Reactions of Dolabelladienones. Total Synthesis of (+)-Fusicoauritone, Angew Chem. Int. Ed. 2007, 46, 915–918. [PDF]

Investigations of Pd-Catalyzed Aryl Substitution Reactions. A Case Study Towards Zoanthenol
D. R. Williams, D. C. Ihle, T. A. Brugel and S. Patnaik, Investigations of Pd-Catalyzed Aryl Substitution Reactions. A Case Study Towards Zoanthenol, Heterocycles, 2006, 70, 77–82 (S. Weinreb Special Issue).
![Studies of the Generation and Pericyclic Behavior of Cyclic Pentadienyl Carbanions. Alkylation Reactions As an Efficient Route to Functionalized cis-Bicyclo[3.3.0]octenes](https://williams.lab.indiana.edu/wp-content/plugins/ezpublications//assets/images/default-pic.jpg)
Studies of the Generation and Pericyclic Behavior of Cyclic Pentadienyl Carbanions. Alkylation Reactions As an Efficient Route to Functionalized cis-Bicyclo[3.3.0]octenes
D. R. Williams, J. T. Reeves, P. P. Nag, W. H. Pitcock, Jr. and M.-H. Baik, Studies of the Generation and Pericyclic Behavior of Cyclic Pentadienyl Carbanions. Alkylation Reactions As an Efficient Route to Functionalized cis-Bicyclo[3.3.0]octenes, J. Am. Chem. Soc. 2006, 128, 12339–12348.

Reactivity Studies of 3,3-Bis(trimethylsilyl)-2-methyl-1-propene in Lewis Acid-Catalyzed Allylation Reactions
D. R. Williams, Á. I. Morales-Ramos and C. M. Williams, Reactivity Studies of 3,3-Bis(trimethylsilyl)-2-methyl-1-propene in Lewis Acid-Catalyzed Allylation Reactions, Org. Lett. 2006, 8, 4393–4396.

An Enantiocontrolled Approach for the Synthesis of Chiral 3,5-Disubstituted-2(1H)-Pyridinones
D. R. Williams, D. C. Kammler and W. R. F. Goundry, An Enantiocontrolled Approach for the Synthesis of Chiral 3,5-Disubstituted-2(1H)-Pyridinones, Heterocycles, 2006, 67, 555–559 (B. Trost Special Issue).

Samarium Diiodide-Mediated Barbier Additions of Iodomethyl Heterocycles to Aldehydes
D. R. Williams and B. W. Stroup, Samarium Diiodide-Mediated Barbier Additions of Iodomethyl Heterocycles to Aldehydes, Org. Lett. 2005, 7, 4099–4102.

Studies Toward the Synthesis of Kendomycin
D. R. Williams and K. Shamim, Studies Toward the Synthesis of Kendomycin, Org. Lett. 2005, 7, 4161–4164.

1-Alkoxyallene as an Effective Precursor for Regio- and Stereocontrolled Allylation Reactions with Aliphatic Aldehydes via Bis-Stannylation
D. R. Williams and M. W. Fultz, 1-Alkoxyallene as an Effective Precursor for Regio- and Stereocontrolled Allylation Reactions with Aliphatic Aldehydes via Bis-Stannylation, J. Am. Chem. Soc. 2005, 127, 14550–14551.

Samarium Diiodide-Mediated Barbier Additions of Iodomethyl Heterocycles to Aldehydes
D. R. Williams and B. W. Stroup, Samarium Diiodide-Mediated Barbier Additions of Iodomethyl Heterocycles to Aldehydes, Org. Lett. 2005, 7, 4099–4102.

Total Synthesis of (+)-Apiosporamide: Assignment of Relative and Absolute Configuration
D. R. Williams, D. C. Kammler, A. F. Donnell and W. R. F. Goundry, Total Synthesis of (+)-Apiosporamide: Assignment of Relative and Absolute Configuration, Angew. Chem. Int. Ed. 2005, 44, 6715–6718.
![Carbolithiation for the Generation of Cyclooctadienyl Anions and Tandem Electrocyclization/Alkylation to Functionalized cis-Bicyclo[3.3.0]octenes](https://williams.lab.indiana.edu/wp-content/plugins/ezpublications//assets/images/default-pic.jpg)
Carbolithiation for the Generation of Cyclooctadienyl Anions and Tandem Electrocyclization/Alkylation to Functionalized cis-Bicyclo[3.3.0]octenes
D. R. Williams and J. T. Reeves, Carbolithiation for the Generation of Cyclooctadienyl Anions and Tandem Electrocyclization/Alkylation to Functionalized cis-Bicyclo[3.3.0]octenes, J. Am. Chem. Soc. 2004, 126, 3434–3435.

Studies of Stereocontrolled Allylation Reactions for the Total Synthesis of Phorboxazole A
D. R. Williams, A. A. Kiryanov, U. Emde, M. P. Clark, M. A. Berliner and J. T. Reeves, Studies of Stereocontrolled Allylation Reactions for the Total Synthesis of Phorboxazole A, Proc. Natl. Acad. Sci. U.S.A. 2004, 101, 12058–12063. [PDF]

Asymmetric Conjugate Addition for the Preparation of syn-1,3-Dimethyl Arrays: Synthesis and Structure Elucidation of Capensifuranone
D. R. Williams, A. L. Nold and R. J. Mullins, Asymmetric Conjugate Addition for the Preparation of syn-1,3-Dimethyl Arrays: Synthesis and Structure Elucidation of Capensifuranone, J. Org. Chem. 2004, 69, 5374–5382.

Chapter 5.4: Paal Thiophene Synthesis in Name Reactions in Heterocyclic Chemistry
D. R. Williams and R. J. Mullins, Chapter 5.4: Paal Thiophene Synthesis in Name Reactions in Heterocyclic Chemistry, Li, J. J., Ed.; Wiley: New York, 2004, pp. 207–217.

Chapter 5.3: Hinsberg Synthesis of Thiophene Derivatives in Name Reactions in Heterocyclic Chemistry
D. R. Williams and R. J. Mullins, Chapter 5.3: Hinsberg Synthesis of Thiophene Derivatives in Name Reactions in Heterocyclic Chemistry, Li, J. J., Ed.; Wiley: New York, 2004, pp. 199–206.

Chapter 5.1: Fiesselmann Thiophene Synthesis in Name Reactions in Heterocyclic Chemistry
D. R. Williams and R. J. Mullins, Chapter 5.1: Fiesselmann Thiophene Synthesis in Name Reactions in Heterocyclic Chemistry, Li, J. J., Ed.; Wiley: New York, 2004, pp. 184–192.

Diastereoselectivity in Asymmetric Allylations. The Role of Vicinal Chirality in the Allyl Nucleophile for SE2” Reactions with Aldehydes
D. R. Williams, K. G. Meyer, K. Shamim and S. Patnaik, Diastereoselectivity in Asymmetric Allylations. The Role of Vicinal Chirality in the Allyl Nucleophile for SE2‘‘ Reactions with Aldehydes, Can. J. Chem. 2004, 82, 120–130.

Aldol Reactions of Unsubstituted β-Lactams. Studies of a β-Glycine Enolate Equivalent
D. R. Williams, A. F. Donnell and D. C. Kammler, Aldol Reactions of Unsubstituted β-Lactams. Studies of a β-Glycine Enolate Equivalent, Heterocycles, 2004, 62, 167–172 (L. Paquette Special Issue).

Leucascandrolide A: A Second Generation Formal Synthesis
D. R. Williams, S. Patnaik and S. V. Plummer, Leucascandrolide A: A Second Generation Formal Synthesis, Org. Lett. 2003, 5, 5035–5038.

Total Synthesis of (–)-Stemonine
D. R. Williams, K. Shamim, J. P. Reddy, G. A. Amato and S. M. Shaw, Total Synthesis of (–)-Stemonine, Org. Lett. 2003, 5, 3361–3364.

Asymmetric Conjugate Addition Reactions of Allyl- and Crotylstannanes
D. R. Williams, R. J. Mullins and N. A. Miller, Asymmetric Conjugate Addition Reactions of Allyl- and Crotylstannanes, Chem. Commun. 2003, 2220–2221.

Formal Synthesis of Leucascandrolide A
D. R. Williams, S. V. Plummer and S. Patnaik, Formal Synthesis of Leucascandrolide A, Angew. Chem. Int. Ed. 2003, 42, 3934–3938.

Total Synthesis of Phorboxazole A
D. R. Williams, A. A. Kiryanov, U. Emde, M. P. Clark, M. A. Berliner and J. T. Reeves, Total Synthesis of Phorboxazole A, Angew. Chem. Int. Ed. 2003, 42, 1258–1262.

Total Synthesis of (+)-4,5-Deoxyneodolabelline
D. R. Williams and R. W. Heidebrecht, Jr., Total Synthesis of (+)-4,5-Deoxyneodolabelline, J. Am. Chem. Soc. 2003, 125, 1843–1850.
![(4R,5R)-2-Bromo-1,3-bis[(4-methylphenyl)sulfonyl]-4,5-diphenyl-1,3,2-diazoborolidine, Electronic Encyclopedia of Reagents for Organic Synthesis](https://williams.lab.indiana.edu/wp-content/plugins/ezpublications//assets/images/default-pic.jpg)
(4R,5R)-2-Bromo-1,3-bis[(4-methylphenyl)sulfonyl]-4,5-diphenyl-1,3,2-diazoborolidine, Electronic Encyclopedia of Reagents for Organic Synthesis
D. R. Williams and D. C. Kammler, (4R,5R)-2-Bromo-1,3-bis[(4-methylphenyl)sulfonyl]-4,5-diphenyl-1,3,2-diazoborolidine, Electronic Encyclopedia of Reagents for Organic Synthesis, Paquette, L., Rigby, J., Roush, W. and Wipf, P., Eds.; John Wiley & Sons Ltd.: Sussex, 2003.

Synthesis of (–)-Laulimalide: An Agent for Microtubule Stabilization
D. R. Williams, L. Mi, R. J. Mullins and R. E. Stites, Synthesis of (–)-Laulimalide: An Agent for Microtubule Stabilization, Tetrahedron Lett. 2002, 43, 4841–4844.

Stereoselective Synthesis of Syn- and Anti-1,3- and 1,2-Dimethyl Arrays via Asymmetric Conjugate Additions
D. R. Williams, W. S. Kissel, J. J. Li and R. J. Mullins, Stereoselective Synthesis of Syn– and Anti-1,3- and 1,2-Dimethyl Arrays via Asymmetric Conjugate Additions, Tetrahedron Lett. 2002, 43, 3723–3727.

Zirconium Cation Coordination in the Borohydride-Mediated Synthesis of β-Hydroxy-N-Alkoxylamines
D. R. Williams, J. W. Benbow, T. R. Sattleberg and D. C. Ihle, Zirconium Cation Coordination in the Borohydride-Mediated Synthesis of β-Hydroxy-N-Alkoxylamines, Tetrahedron Lett. 2001, 42, 8597–8601.

Total Synthesis of Cystothiazoles A and C
D. R. Williams, S. Patnaik and M. P. Clark, Total Synthesis of Cystothiazoles A and C, J. Org. Chem. 2001, 66, 8463–8469.

Total Synthesis of (–)-Stemospironine
D. R. Williams, M. G. Fromhold and J. D. Earley, Total Synthesis of (–)-Stemospironine, Org. Lett. 2001, 3, 2721–2724.

Total Synthesis of (–)-Ratjadone
D. R. Williams, D. C. Ihle and S. V. Plummer, Total Synthesis of (–)-Ratjadone, Org. Lett. 2001, 3, 1383–1386.

Total Synthesis of (+)-Amphidinolide K
D. R. Williams and K. G. Meyer, Total Synthesis of (+)-Amphidinolide K, J. Am. Chem. Soc. 2001, 123, 765–767.

Advancements in Chemical Synthesis: An Overview of Studies of Antibiotics from Myxobacteria
D. R. Williams, J. J. Li and R. H. Hutchings, Advancements in Chemical Synthesis: An Overview of Studies of Antibiotics from Myxobacteria, Org. Prep. Proced. Int. 2000, 32, 409–451.

A Synthesis of 3’O,4’O-Dimethylfuniculosin
D. R. Williams, P. D. Lowder and Y.-G. Gu, Studies of Novel Cyclitols. A Synthesis of 3‘O,4‘O-Dimethylfuniculosin, Tetrahedron Lett. 2000, 41, 9397–9401.

Total Synthesis of Lankacyclinol
D. R. Williams, G. S. Cortez, S. L. Bogen and C. M. Rojas, Total Synthesis of Lankacyclinol, Angew. Chemie, Intl. Ed. 2000, 39, 4612–4615.

The Construction of 4-Hydroxy-2-Pyridinones. Total Synthesis of (+)-Sambutoxin
D. R. Williams and R. A. Turske, The Construction of 4-Hydroxy-2-Pyridinones. Total Synthesis of (+)-Sambutoxin, Org. Lett. 2000, 2, 3217–3220.

Synthetic Studies Toward Phorboxazole A. Stereoselective Synthesis of the C28–C46 Side Chain Fragment
D. R. Williams, M. P. Clark, U. Emde and M. A. Berliner, Synthetic Studies Toward Phorboxazole A. Stereoselective Synthesis of the C28–C46 Side Chain Fragment, Org. Lett. 2000, 2, 3023–3026.

The Synthesis of Functionalized Oxazolines and Oxazoles with DAST and Deoxo-Fluor
A. J. Phillips, Y. Uto, P. Wipf, M. J. Reno and D. R. Williams, The Synthesis of Functionalized Oxazolines and Oxazoles with DAST and Deoxo-Fluor, Org. Lett. 2000, 2, 1165–1168.

Intramolecular Diels-Alder Cyclization of E-1-Nitro-1,7,9-Decatrienes: Synthesis of the AB Ring System of Norzoanthamine
D. R. Williams and T. A. Brugel, Intramolecular Diels-Alder Cyclization of E-1-Nitro-1,7,9-Decatrienes: Synthesis of the AB Ring System of Norzoanthamine, Org. Lett. 2000, 2, 1023–1026.

Total Synthesis of (–)-Amphidinolide P
D. R. Williams, B. J. Myers and L. Mi, Total Synthesis of (–)-Amphidinolide P, Org. Lett, 2000, 2, 945–948.

Palladium-Induced Cyclizations for the Synthesis of cis-2,5-Disubstituted-3-Methylene-Tetrahydrofurans: Studies of the C7–C22 Core of Amphidinolide K
D. R. Williams and K. G. Meyer, Palladium-Induced Cyclizations for the Synthesis of cis-2,5-Disubstituted-3-Methylene-Tetrahydrofurans: Studies of the C7–C22 Core of Amphidinolide K, Org. Lett. 1999, 1, 1303–1305.

Total Synthesis of (–)-Hennoxazole A
D. R. Williams, D. A. Brooks and M. A. Berliner, Total Synthesis of (–)-Hennoxazole A, J. Am. Chem. Soc. 1999, 121, 4924–4925.

The Macrocyclic Domain of Phorboxazole A. A Stereoselective Synthesis of the C1–C32 Macrolactone
D. R. Williams and M. P. Clark, The Macrocyclic Domain of Phorboxazole A. A Stereoselective Synthesis of the C1–C32 Macrolactone, Tetrahedron Lett. 1999, 40, 2291–2294.

Synthetic Studies Toward Phorboxazole A. Stereoselective Synthesis of the C3–C19 and C20–C32 Subunits
D. R. Williams, M. P. Clark and M. A. Berliner, Synthetic Studies Toward Phorboxazole A. Stereoselective Synthesis of the C3–C19 and C20–C32 Subunits, Tetrahedron Lett. 1999, 40, 2287–2290.

Studies of Acyl Nitrene Insertions. A Stereocontrolled Route Toward Lankacidin Antibiotics
D. R. Williams, C. M. Rojas and S. L. Bogen, Studies of Acyl Nitrene Insertions. A Stereocontrolled Route Toward Lankacidin Antibiotics, J. Org. Chem. 1999, 64, 736–746.
![Regioselective Ring Metalation in [2,4]-Bisoxazoles](https://williams.lab.indiana.edu/wp-content/plugins/ezpublications//assets/images/default-pic.jpg)
Regioselective Ring Metalation in [2,4]-Bisoxazoles
D. R. Williams, D. A. Brooks, K. G. Meyer and M. Pagel, Regioselective Ring Metalation in [2,4]-Bisoxazoles, Tetrahedron Lett. 1998, 39, 8023–8026.

Diastereoselection in the Conjugate Additions of Organocopper Reagents to N-Enoyloxazolidinones
D. R. Williams, W. S. Kissel and J. Li, Diastereoselection in the Conjugate Additions of Organocopper Reagents to N-Enoyloxazolidinones, Tetrahedron Lett. 1998, 39, 8593–8596.

Total Synthesis of (+)-Amphidinolide J
D. R. Williams and W. S. Kissel, Total Synthesis of (+)-Amphidinolide J, J. Am. Chem. Soc. 1998, 120, 11198–11199.

Synthesis of Atovaquone
D. R. Williams and M. P. Clark, Synthesis of Atovaquone, Tetrahedron Lett. 1998, 39, 7629–7632.

Asymmetric Allylation. An Effective Strategy for the Convergent Synthesis of Highly Functionalized Homoallylic Alcohols
D. R. Williams, D. A. Brooks, K. G. Meyer, and M. P. Clark, Asymmetric Allylation. An Effective Strategy for the Convergent Synthesis of Highly Functionalized Homoallylic Alcohols, Tetrahedron Lett. 1998, 39, 7251–7254.

Studies of Zoanthamine Alkaloids. Enantiocontrolled Construction of the Tetracyclic Hemi-Aminal Core
D. R. Williams and G. S. Cortez, Studies of Zoanthamine Alkaloids. Enantiocontrolled Construction of the Tetracyclic Hemi-Aminal Core, Tetrahedron Lett. 1998, 39, 2675–2678.
![1-[2'-(Trimethylsilyl)ethoxymethyl]-2-phenylsulfonylimidazole: A New Reagent for the Preparation of C-4 Imidazoles](https://williams.lab.indiana.edu/wp-content/plugins/ezpublications//assets/images/default-pic.jpg)
1-[2′-(Trimethylsilyl)ethoxymethyl]-2-phenylsulfonylimidazole: A New Reagent for the Preparation of C-4 Imidazoles
J. G. Phillips, L. Fadnis and D. R. Williams, 1-[2‘-(Trimethylsilyl)ethoxymethyl]-2-phenylsulfonylimidazole: A New Reagent for the Preparation of C-4 Imidazoles, Tetrahedron Lett. 1997, 38, 7835–7838.

Total Synthesis of Rhizoxin D
D. R. Williams, K. M. Werner and B. Feng, Total Synthesis of Rhizoxin D, Tetrahedron Lett. 1997, 38, 6825–6828.

Enantioselective Alkylation of Aldehydes with Diethylzinc Catalyzed by C2-Symmetric Ligands
D. R. Williams and M. G. Fromhold, Enantioselective Alkylation of Aldehydes with Diethylzinc Catalyzed by C2-Symmetric Ligands, Synlett, 1997, 5, 523–524.

Studies of Mild Dehydrogenations in Heterocyclic Systems
D. R. Williams, P. D. Lowder, Y.-G. Gu and D. A. Brooks, Studies of Mild Dehydrogenations in Heterocyclic Systems, Tetrahedron Lett. 1997, 38, 331–334.

Studies Toward Funiculosin. Intramolecular Carbonyl Condensations Using Carboxamidimidazolide Intermediates
D. R. Williams, P. D. Lowder and Y.-G. Gu, Studies Toward Funiculosin. Intramolecular Carbonyl Condensations Using Carboxamidimidazolide Intermediates, Tetrahedron Lett. 1997, 38, 327–330.

The Preparation and Wittig Condensations of C-4 Thiazole Phosphonium Methylides
D. R. Williams, D. A. Brooks, J. L. Moore and A. O. Stewart, The Preparation and Wittig Condensations of C-4 Thiazole Phosphonium Methylides, Tetrahedron Lett. 1996, 37, 983–986.

Studies on the Dolabellanes: Stereoselective Transannular Cyclizations of Dolabelladiene Macrocycles
D. R. Williams and P. J. Coleman, Studies on the Dolabellanes: Stereoselective Transannular Cyclizations of Dolabelladiene Macrocycles, Tetrahedron Lett. 1995, 36, 39–42.

Total Synthesis of Neodolabellenol
D. R. Williams and P. J. Coleman, Total Synthesis of Neodolabellenol, Tetrahedron Lett. 1995, 36, 35–38.
![Functionalization and Utility of Bridging Ethers in the Transformations of Bicyclo[5.4.0]undecanes](https://williams.lab.indiana.edu/wp-content/plugins/ezpublications//assets/images/default-pic.jpg)
Functionalization and Utility of Bridging Ethers in the Transformations of Bicyclo[5.4.0]undecanes
D. R. Williams, J. W. Benbow, J. G. McNutt and E. E. Allen, Functionalization and Utility of Bridging Ethers in the Transformations of Bicyclo[5.4.0]undecanes, J. Org. Chem. 1995, 60, 833–843.

Total Synthesis of (–)-Stemoamide
D. R. Williams, J. P. Reddy and G. S. Amato, Total Synthesis of (–)-Stemoamide, Tetrahedron Lett. 1994, 35, 6417–6420.

Total Synthesis of Myxovirescin A1
D. R. Williams and J. Li, Total Synthesis of Myxovirescin A1, Tetrahedron Lett. 1994, 35, 5113–5116.

Synthesis Strategies for Marine Diterpenes. Total Synthesis of the Clavularanes
D. R. Williams, P. J. Coleman and S. S. Henry, Synthesis Strategies for Marine Diterpenes. Total Synthesis of the Clavularanes, J. Am. Chem. Soc. 1993, 115, 11654–11655.

An Efficient Synthesis of the Dolabellanes
D. R. Williams, P. J. Coleman, C. R. Nevill and L. A. Robinson, An Efficient Synthesis of the Dolabellanes, Tetrahedron Lett. 1993, 34, 7895–7898.

Carbanion Methodology for Alkylations and Acylations in the Synthesis of Substituted Oxazoles. The Formation of Cornforth Rearrangement Products
D. R. Williams and E. L. McClymont, Carbanion Methodology for Alkylations and Acylations in the Synthesis of Substituted Oxazoles. The Formation of Cornforth Rearrangement Products, Tetrahedron Lett. 1993, 34, 7705–7708.

Diastereoselective Hydride Reductions of α-Hydroxy Oximino Ethers. Synthesis of syn-1,2-Amino Alcohols
D. R. Williams, M. H. Osterhout and J. P. Reddy, Diastereoselective Hydride Reductions of α-Hydroxy Oximino Ethers. Synthesis of syn-1,2-Amino Alcohols, Tetrahedron Lett. 1993, 34, 3271–3274.

A Study of Stereocontrol in Spiroketalizations. The Role of Hydroxy-Assisted Chelation Control
D. R. Williams, P. A. Jass and R. D. Gaston, A Study of Stereocontrol in Spiroketalizations. The Role of Hydroxy-Assisted Chelation Control, Tetrahedron Lett. 1993, 34, 3231–3234.

Stereocontrolled Hydride Reductions of β-Hydroxy Oximino-Ethers
D. R. Williams and M. H. Osterhout, Stereocontrolled Hydride Reductions of β-Hydroxy Oximino-Ethers, J. Am. Chem. Soc. 1992, 114, 8750–8751.

A Preparation of Unsymmetrical α-Diketones
D. R. Williams, L. A. Robinson, G. S. Amato and M. H. Osterhout, A Preparation of Unsymmetrical α-Diketones, J. Org. Chem. 1992, 57, 3740–3744.

Total Synthesis of (+)-Breynolide
D. R. Williams, P. A. Jass, H.-L. A. Tse and R. D. Gaston, Total Synthesis of (+)-Breynolide, J. Am. Chem. Soc. 1990, 112, 4552–4554.

Intramolecular Dipolar Addition of Carbonyl Ylides. Studies of Substituted Bicycloundecanones
D. R. Williams, J. W. Benbow and E. E. Allen, Intramolecular Dipolar Addition of Carbonyl Ylides. Studies of Substituted Bicycloundecanones, Tetrahedron Lett. 1990, 31, 6769–6772.

Total Synthesis of (+)-Myxovirescin B
D. R. Williams and J. M. McGill, Total Synthesis of (+)-Myxovirescin B, J. Org. Chem. 1990, 55, 3457–3459.

Alkylation Studies of Anions from Pyruvate Oximes and Related Derivatives
D. R. Williams and J. W. Benbow, Alkylation Studies of Anions from Pyruvate Oximes and Related Derivatives, Tetrahedron Lett. 1990, 31, 5881–5884.

Synthesis of the “Tricarbonyl” Region of FK-506 Through an Amidophosphorane
H. H. Wasserman, V. M. Rotello, D. R. Williams and J. W. Benbow, Synthesis of the “Tricarbonyl” Region of FK-506 Through an Amidophosphorane, J. Org. Chem. 1989, 54, 2785–2786.

Studies of Stemona Alkaloids. Total Synthesis of (+)-Croomine
D. R. Williams, D. L. Brown and J. W. Benbow, Studies of Stemona Alkaloids. Total Synthesis of (+)-Croomine, J. Am. Chem. Soc. 1989, 111, 1923–1925.

Anchimeric Assistance With Intermediary N-Alkoxyaziridinium Salts. Formation of Vicinal Aminoalcohols and Derivatives
D. R. Williams, M. H. Osterhout and J. M. McGill, Anchimeric Assistance With Intermediary N-Alkoxyaziridinium Salts. Formation of Vicinal Aminoalcohols and Derivatives, Tetrahedron Lett. 1989, 30, 1331–1334.

Intramolecular Cyclizations from N-Alkoxyamines. Formation of Dialkylsubstituted Pyrrolidines and Piperidines
D. R. Williams, M. H. Osterhout and J. M. McGill, Intramolecular Cyclizations from N-Alkoxyamines. Formation of Dialkylsubstituted Pyrrolidines and Piperidine s, Tetrahedron Lett. 1989, 30, 1327–1330.

Explorations Toward Pseudomonic Acid C
D. R. Williams, Explorations Toward Pseudomonic Acid C, Strategies and Tactics in Organic Synthesis, Vol. 2, Lindberg, T., Ed.; Academic Press: San Diego, 1989, pp. 439–464.

Synthesis of the α,β-Diketoamide Segment of the Novel Immunosuppressive FK506
D. R. Williams and J. W. Benbow, Synthesis of the α,β-Diketoamide Segment of the Novel Immunosuppressive FK506, J. Org. Chem. 1988, 53, 4643–4644.

Bromine As An Oxidant for Direct Conversion of Aldehydes to Esters
D. R. Williams, F. D. Klingler, E. E. Allen and F. W. Lichtenthaler, Bromine As An Oxidant for Direct Conversion of Aldehydes to Esters, Tetrahedron Lett. 1988, 29, 5087–5090.

Synthesis of the Optically Active Hexahydrobenzofuran Nucleus of the Avermectins
D. R. Williams, F. D. Klingler and V. Dabral, Synthesis of the Optically Active Hexahydrobenzofuran Nucleus of the Avermectins, Tetrahedron Lett. 1988, 29, 3415–3418.

Intramolecular Claisen Condensations: An Efficient Route Toward the Avermectins and Milbemycins
D. R. Williams and F. D. Klingler, Intramolecular Claisen Condensations: An Efficient Route Toward the Avermectins and Milbemycins, J. Org. Chem. 1988, 53, 2134–2136.

Total Synthesis of (+)-Citreoviridin
D. R. Williams and F. H. White, Total Synthesis of (+)-Citreoviridin, J. Org. Chem. 1987, 52, 5067–5079.

A General Preparation of α-Alkoxyacroleins
D. R. Williams, R. D. Gaston, and J. F. Hoover, A General Preparation of α-Alkoxyacroleins, Synthesis, 1987, 10, 908–910.

Stereoselective Allylation for Preparation of L-Hexose Derivatives
D. R. Williams and F. D. Klingler, Stereoselective Allylation for Preparation of L-Hexose Derivatives, Tetrahedron Lett. 1987, 28, 869–872.

Total Synthesis of (+)-Pseudomonic Acid C
D. R. Williams, J. L. Moore and M. Yamada, Total Synthesis of (+)-Pseudomonic Acid C, J. Org. Chem. 1986, 51, 3916–3918.

Studies of Tetrasubstituted Tetrahydrofurans
D. R. Williams and F. H. White, Studies of Tetrasubstituted Tetrahydrofurans, Tetrahedron Lett. 1986, 27, 2195–2198.

Intramolecular Cycloadditions Using Vinyl Sulfide Dienophiles
D. R. Williams and R. D. Gaston, Intramolecular Cycloadditions Using Vinyl Sulfide Dienophiles, Tetrahedron Lett. 1986, 27, 1485–1488.

A Route to Stereochemically Complex Tetrahydrofurans Using α-Sulfinyl Carbanions
D. R. Williams and J. G. Phillips, A Route to Stereochemically Complex Tetrahydrofurans Using α-Sulfinyl Carbanions, Tetrahedron, 1986, 42, 3013–3019.

Studies of Stereochemical Control Using α-Lithiosulfinyl Carbanions
D. R. Williams, J. G. Phillips, F. H. White and J. C. Huffman, Studies of Stereochemical Control Using α-Lithiosulfinyl Carbanions, Tetrahedron, 1986, 42, 3003–3011.

Hydroxyl-Directed Iodoetherifications of Allylic Alcohols. Synthesis of (+)-Citreoviral,
D. R. Williams and F. H. White, Hydroxyl-Directed Iodoetherifications of Allylic Alcohols. Synthesis of (+)-Citreoviral, Tetrahedron Lett. 1985, 26, 2529–2532.

Total Synthesis of (+)-Ilicicolin H
D. R. Williams, M. L. Bremmer, D. L. Brown and J. D’Antuono, Total Synthesis of (+)-Ilicicolin H, J. Org Chem. 1985, 50, 2807–2809.

Intramolecular Diels-Alder Cycloaddition of Bis-Diene Substrates
D. R. Williams, R. D. Gaston and I. B. Horton, III, Intramolecular Diels-Alder Cycloaddition of Bis-Diene Substrates, Tetrahedron Lett. 1985, 26, 1391–1394.

Stereocontrol in the Formation of 2,3,4-Trisubstituted Tetrahydrofurans
D. R. Williams, J. Grote and Y. Harigaya, Stereocontrol in the Formation of 2,3,4-Trisubstituted Tetrahydrofurans, Tetrahedron Lett. 1984, 25, 5231–5234.

Total Synthesis of (+)-Phyllanthocin
D. R. Williams and S.-Y. Sit, Total Synthesis of (+)-Phyllanthocin, J. Am. Chem. Soc. 1984, 106, 2949–2954.

Stereocontrolled Transformations of Orthoester Intermediates into Substituted Tetrahydrofurans
D. R. Williams, Y. Harigaya, J. L. Moore and A. D’sa, Stereocontrolled Transformations of Orthoester Intermediates into Substituted Tetrahydrofurans, J. Am. Chem. Soc. 1984, 106, 2641–2644.

Opportunities for Selective Removal of Methoxyethoxymethyl (MEM) Ethers
D. R. Williams and S. Sakdarat, Opportunities for Selective Removal of Methoxyethoxymethyl (MEM) Ethers, Tetrahedron Lett. 1983, 24, 3965–3968.

Ring Formation by Base-Dependent Isomerizations of Epoxybenzyl Ethers
D. R. Williams and J. Grote, Ring Formation by Base-Dependent Isomerizations of Epoxybenzyl Ethers, J. Org. Chem. 1983, 48, 134–136.

Thione Reductions for Preparation of Five-Membered Heterocycles
D. R. Williams and J. L. Moore, Thione Reductions for Preparation of Five-Membered Heterocycles, Tetrahedron Lett. 1983, 24, 339–342.
![Synthetic Studies of 1,7-Dioxaspiro[5.5]Undecan-4-Ones](https://williams.lab.indiana.edu/wp-content/plugins/ezpublications//assets/images/default-pic.jpg)
Synthetic Studies of 1,7-Dioxaspiro[5.5]Undecan-4-Ones
D. R. Williams and B. A. Barner, Synthetic Studies of 1,7-Dioxaspiro[5.5]Undecan-4-Ones, Tetrahedron Lett. 1983, 24, 427–430.

Total Synthesis of Milbemycin β3
D. R. Williams, B. A. Barner, K. Nishitani and J. G. Phillips, Total Synthesis of Milbemycin β3, J. Am. Chem. Soc. 1982, 104, 4708–4710.

Synthesis of Racemic Tenellin
D. R. Williams and S.-Y. Sit, Synthesis of Racemic Tenellin, J. Org. Chem. 1982, 47, 2846–2851.

A Synthesis of the Juvabiols
D. R. Williams and J. G. Phillips, A Synthesis of the Juvabiols, J. Org. Chem. 1981, 46, 5452–5454.

A Stereospecific Synthesis of Highly Substituted Tetrahydrofurans
D. R. Williams, J. G. Phillips and B. A. Barner, A Stereospecific Synthesis of Highly Substituted Tetrahydrofurans, J. Am. Chem. Soc. 1981, 103, 7398–7399.

The Formation of Cyclopentenones and 3 (2H)-Furanones from Acetylenic Precursors
D. R. Williams, A. Abbaspour and R. M. Jacobson, The Formation of Cyclopentenones and 3 (2H)-Furanones from Acetylenic Precursors, Tetrahedron Lett. 1981, 22, 3565–3568.

A Preparation of Bromoolefins from Carbonyl Compounds
D. R. Williams, K. Nishitani, W. Bennett and S.-Y. Sit, A Preparation of Bromoolefins from Carbonyl Compounds, Tetrahedron Lett. 1981, 22, 3745–3748.

A Stereoselective Approach to Acyclic Systems via Condensation at α-Lithiosulfinyl Carbanions and Aldehydes
D. R. Williams, J. G. Phillips and J. C. Huffman, A Stereoselective Approach to Acyclic Systems via Condensation at α-Lithiosulfinyl Carbanions and Aldehydes, J. Org. Chem. 1981, 46, 4101–4103.

A Mild Oxidation of Aldehydes to α,β-Unsaturated Aldehydes
D. R. Williams and K. Nishitani, A Mild Oxidation of Aldehydes to α,β-Unsaturated Aldehydes, Tetrahedron Lett. 1980, 22, 4417–4420.

Total Synthesis of (+)-Brefeldin A
E. J. Corey, R. M. Wollenberg and D. R. Williams, Total Synthesis of (+)-Brefeldin A, Tetrahedron Lett. 1977, 26, 2243–2246.

A Total Synthesis of (+)-Cerulenin
E. J. Corey and D. R. Williams, A Total Synthesis of (+)-Cerulenin, Tetrahedron Lett. 1977, 44, 3847–3850.

Synthesis of 2-Substituted, 3-Dimethylamino-5,6-methylenedioxyindenes and the Corresponding Indanones
D. T. Witiak, D. R. Williams and S. V. Kakodkar, Vilsmeier-Haack Cyclizations: Synthesis of 2-Substituted, 3-Dimethylamino-5,6-methylenedioxyindenes and the Corresponding Indanones, J. Org. Chem. 1974, 39, 1242–1247.